FAQs From CDMOs About Accessing Life Science Connect Audiences

Buyers are less willing to talk to suppliers than ever before. This is especially true in the life sciences space, where biopharma companies prefer to do their own research instead of talking to a CDMO's sales team.
A biopharma company's buyer’s journeys to select a CDMO is already 50-75% complete before that CDMO knows about a prospect's intent to outsource. So how can CDMOs engage prospects early enough to have a fighting chance? Life Science Connect's answer to that question is audience access.
Audience access is the foundation of a content marketing program. Your content gets in front of our audience when it is hosted on our sites and pushed out through our newsletters.
Here's what that looks like in a nutshell. First, we help you identify a target audience (based on factors like molecule type, pipeline phase, therapeutic area, geography, and much more). Then, we help you select your most impactful content to share with our audience (or we work with you to create that content). Your content is included at least 2x per month in the Industry Insights section of our newsletter, where thousands of biopharma readers can access your thought leadership alongside our original editorial.
Being a successful CDMO marketer hinges on getting your content in front of an engaged audience of your biopharma prospects. To do this, you can’t just buy a list of names or hope the right decision-makers will come to your website. It’s a time consuming, resource-intensive endeavor to build, maintain, and grow an active, high-quality database. The price of audience access covers our cost to do this for you – that way you can focus on filling capacity instead of worrying about things like data integrity, compliance, email deliverability, and list cleaning.
FAQ
What kind of data comes with audience access?
When a reader accesses your content on one of our sites, we automatically capture contact information and data about that interaction. This data doesn’t just get dumped in a spreadsheet – our business development team helps you to understand and act on it.
For each contact interaction, you'll be able to see:
- First and last name
- Title
- Company name
- Company type (varies by market)
- Phone number
- Address
- Document title
- Document type (white paper, e-book, case study, brochure, etc.)
- Buyer's journey stage of the document (early, middle, late)
- Interaction type (content download, share, web search)
- Interaction date and time
Some of our CDMO partners have upgraded programs that allow them to see things like therapeutic area, route of administration, molecule type, etc. in their lead reports. We can provide this data so that your sales and marketing team doesn't have to spend valuable time looking this information up.
How do you capture data? Do readers hit a gate for every content download?
Our readers have registered user profiles. When readers access your content, we capture this activity based on their user profile (according to strict privacy standards that exceed the requirements of GDPR, of course). Readers are prompted to log in to their user profile, or they can access the content directly from a newsletter.
Our proprietary technologies connect biopharma readers to content that helps with their buyer’s journey and then tracks their engagement on the back end.
Or, in simpler terms, when a reader clicks on your content, we’ll tell you about it.
Why can't audience access be purchased on a month-by-month or trial basis?
Even the best content strategy can’t magically shortcut a CDMO's sales cycles. It takes time to be an effective B2B content marketer. It takes time to spot behavior trends and purchase intent from our readers. We believe the minimum amount of time needed to evaluate the effectiveness of your content is six months.
CDMOs typically don’t know when a new buyer’s journey will begin. While large pharma often has a buyer’s journey that aligns with the calendar/budgeting year, small to mid-sized pharma and biopharma has more random buyer’s journeys that are often aligned with the progress of programs as well as funding/partnering events. This makes it difficult to know who has an active project and when they have it. If your content program is "always on," that means companies looking to outsource can always find your expertise, regardless of when they have an active project.
Our typical programs actually suggest a 12-month program. That allows us to help you identify engagement trends to understand the buyer’s journey. Plus, we constantly add new readers to our audience. And those readers are constantly starting new buyer’s journeys. Your best chance to reach as many of these readers as possible is to be in front of them for an extended period.
Think of it this way: You wouldn't plan to build a new, state-of-the-art facility and hire the brightest scientific minds available but then only purchase half of the equipment you need. You either invest in the equipment, technology, and staff expertise you need, or you don't. Life Science Connect strongly believes the same is true about content marketing: You either make a commitment to long-term success, or your marketing won't work.
Why is audience access required to purchase other custom marketing services?
You can increase your share of voice and elevate your status as a thought leader if your content is where readers search for information (on our websites and in our newsletters). Some readers only access content through our regular newsletters, and some find your content on our sites through organic web search. Audience access allows you to continuously capture high-quality leads through newsletters and organic search. At a minimum, you need to have this constant presence to build trust and awareness among our readers.
Think of it like buying a car. The car only works with an engine, which is an engaged audience. That engine only runs if it has fuel, which is your content. You can’t buy the leather seats, the sunroof, and the chrome rims unless you first buy the car itself and start driving down the buyer’s journey path with your content.
How many leads can I expect from an audience access program?
The truth is, it depends. And any media partner that "guarantees" leads likely isn't helping you fully integrate with a biopharma company's buyer's journey. That said, there are a few factors that influence the number of leads you can get, and one of our business development team members can walk you through an estimated target.
Life Science Connect has 10 different communities that cover every stage of drug development from discovery up through commercialization. Depending on who you are trying to target, you might want to access multiple audiences (for example, Outsourced Pharma and Pharmaceutical Online and Cell And Gene).
The number of leads you'll generate will also depend on what type of content you promote to our audiences. Highly-promotional content such as a brochure about your areas of specialization is going to generate fewer leads than a thought leadership article that helps biopharma companies understand regulatory challenges. There is very little crossover between our audiences because we strive to serve our readers the most relevant, tailored content.
Why do I have to purchase audience access for each site?
Think back to the good old days of buying B2B advertising via print magazines. If you wanted to be in magazine A, you bought a full-page ad. If you wanted to be in magazine B, you had to buy a different ad. You can use the same creative, but it's still two separate purchases. Those ads are how you gained access to those print audiences. Think of each of our websites as its own "magazine." BioprocessOnline.com has a different audience than OutsourcedPharma.com than CellandGene.com. Different audiences mean different audience access programs are required for each community.
Audience Access: A Case Study
Background: A reader, in this case a $2 billion pharma company, had an active buyer’s journey to award a multi-million-dollar contract to a CRO for a rare disease phase one clinical trial.
Reader Info: Over six months, a total of 5 people from this pharma company engaged with content on a Life Science Connect publication (ClinicalLeader.com).
Supplier Info: The CRO that was awarded the contract partners with the ClinicalLeader.com platform. We reverse-engineered the sale to take a closer look at how this reader company engaged with supplier content.
Marketing Activities: This CRO had an audience access program and a custom newsletter on ClinicalLeader.com.
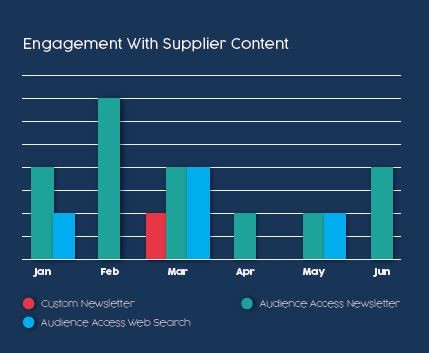
Content Engagements: From January through June, this supplier’s six-month audience access program generated nearly 1,500 engagements from 12 content inclusions in our regular editorial newsletters. This was in addition to 110 content engagements generated by organic web searches that found supplier content posted on our site. During that same six-month period, this supplier also ran one custom newsletter that generated more than 700 content engagements. On the surface, it seems like it’s better to generate 700 leads with one activity compared to generating 1,600 leads from a dozen activities over a six-month timeframe.
But let’s look back at the reader company, a $2 billion pharma company, with an active buyer’s journey to choose a supplier for their phase one clinical trial. In that same six-month window, 5 different people at that pharma company engaged with this supplier’s content.
If the supplier only had custom newsletters instead of the audience access program, this reader engagement would not have indicated an active buyer’s journey. Instead, it would have looked like chart #1 (only one person from the $2 billion pharma company engaging with one piece of content).

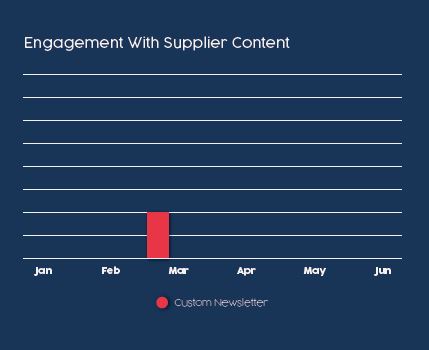
Fortunately, because of the continued engagement generated from the audience access program, the buyer’s journey actually looked like chart #2. A total of 5 people at that $2 billion pharma company had 17 engagements with 6 different pieces of content during these six months. All but 1 of those engagements were attributed to the audience access program.
 Originally published on Follow Your Buyer
Originally published on Follow Your Buyer
Let's work together.
Whether you're ready to hit the ground running or just starting and have questions, we're here to understand your goals and explore how we can help you.